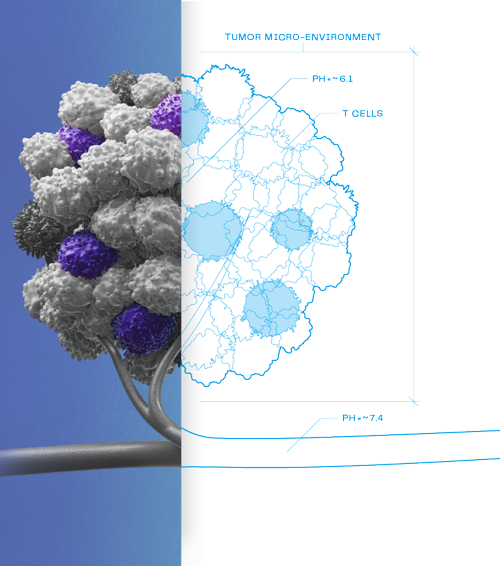
We’ve built a pipeline of investigational therapies that are designed to work selectively in the tumor microenvironment. These conditionally active antibodies are expected to unlock previously undruggable targets and may offer new treatment options for cancer patients.